Stable Cell Line Development offer
Derisk seamlessy your project by starting with a free audit and an In silico sequence developability evaluation
Generating a Stable Cell Line is the vital final step before entering the preclinical or clinical phase. In order to increase chances to achieve high yields, we offer a manufacturability assessment that is designed to anticipate challenges, such as purification difficulties.
What's inside this offer?
1 – An insighful audit of your project
Our journey together starts with a personalized consultation to delve into the unique needs and background of your biologics development project.
Objective: This insightful conversation allows us to craft a tailored roadmap that perfectly aligns with your goals.
2 – In silico sequence developability evaluation thanks to proprietary AI platform
- Analysis of CDRs (complementarity-determining regions)
- Identification of liability sequences (potential degradation or instability issues)
- Solubility evaluation
- Aggregation risk assessment
Objective: To obtain optimized sequences that eliminate manufacturability risks while preserving the key properties of the antibody (affinity, specificity, degree of humanization).
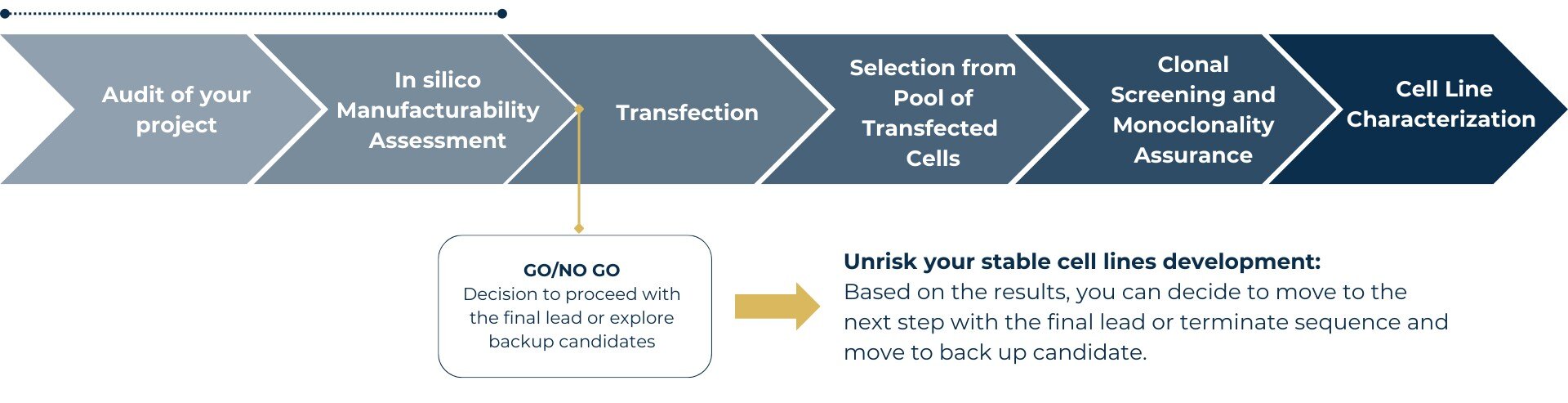
Explore our streamlined workflow for stable cell line development and see how manufacturability assessment ensures project confidence from the start.